Description
Building block with side protection orthogonal to Fmoc/tBu strategy. Can be used for side specific derivatization, for example for TFA mediated in situ cyclization, which is taking advantage of intermediate aspartamide formation. Reference: A Tandem In Situ Peptide Cyclization through Trifluoroacetic Acid Cleavage; Koushik Chandra, Tapta Kanchan Roy, Deborah E. Shalev, Abraham Loyter, Chaim Gilon, R. Benny Gerber, and Assaf Friedler; Angew. Chem. Int. Ed. 2014; 53: 9450-9455. DOI: 10.1002/anie.201402789. N-Allyloxycarbonyl (Alloc) are widely used protecting groups, i.e. for amines and amino acids, as they are quantitatively and very rapidly converted to free amino compounds by palladium. Deprotection of allyl (All) carboxylates and allyl aryl ethers can also be carried out accordingly. All and Alloc are fully compatible with Fmoc/tBu and Boc/Bzl strategies, as well as with Z protecting groups. Selective cleavage of the allyl and (allyloxy)carbonyl groups through palladium-catalyzed hydrostannolysis with tributyltin hydride. Application to the selective protection-deprotection of amino acid derivatives and in peptide synthesis; O. Dangles, F. Guibe, G. Balavoine, S. Lavielle and A. Marquet; The Journal of Organic Chemistry 1987; 52: 4984-4993. doi:10.1021/jo00231a027 Allylic protecting groups and their use in a complex environment part II: Allylic protecting groups and their removal through catalytic palladium p-allyl methodology; F. Guibé; Tetrahedron 1998; 54: 2967-3042. doi:http://dx.doi.org/10.1016/S0040-4020(97)10383-0 Mild non-transition metal catalyzed deprotection of N-allyloxycarbonyl amines; R. H. Szumigala Jr, E. Onofiok, S. Karady, J. D. Armstrong Iii and R. A. Miller; Tetrahedron letters 2005; 46: 4403-4405. doi:http://dx.doi.org/10.1016/j.tetlet.2005.04.064 Highly efficient removal of allyloxycarbonyl (Alloc) function provides a practical orthogonal protective strategy for carbohydrates; G. H. Zong, S. Q. Yan, X. M. Liang, J. J. Zhang, D. Q. Wang and F. Z. Kong; Chinese Chemical Letters 2009; 20: 127-130. doi:http://dx.doi.org/10.1016/j.cclet.2008.11.002 The Allyloxycarbonyl (Aloc) Moiety-Conversion of an Unsuitable into a Valuable Amino Protecting Group for Peptide Synthesis; H. Kunz and C. Unverzagt; Angewandte Chemie International Edition in English 1984; 23: 436-437. doi:10.1002/anie.198404361
x
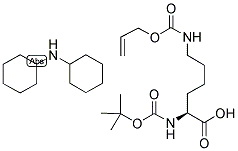
Alternative Distributors of [N-alpha-t-Butyloxycarbonyl-N-epsilon-allyloxycarbonyl-L-lysine dicyclohexylamine]
Producers or manufacturers change the product range from time to time.
The following companies
have appeared as suppliers in the past / currently not verified
ABCR GmbH & Co. KG | Jinan Haohua Industry Co., Ltd.



 Chemical-Suppliers.eu
Chemical-Suppliers.eu